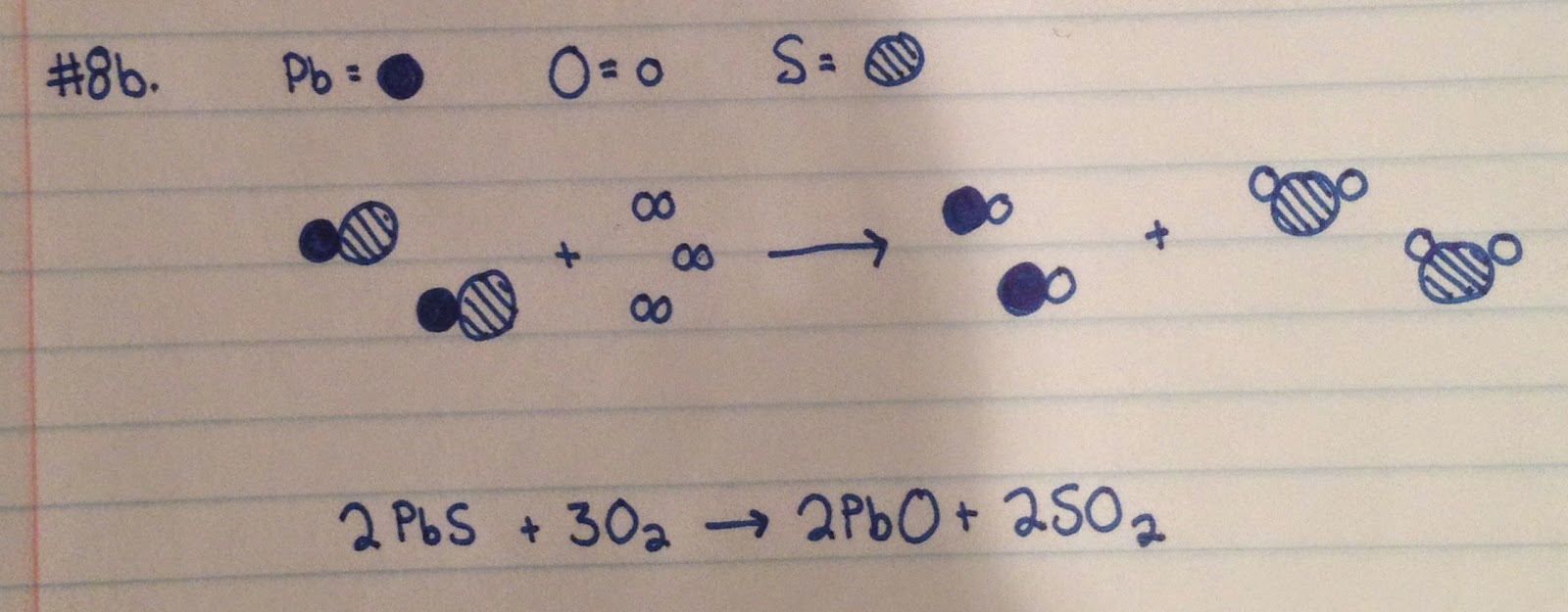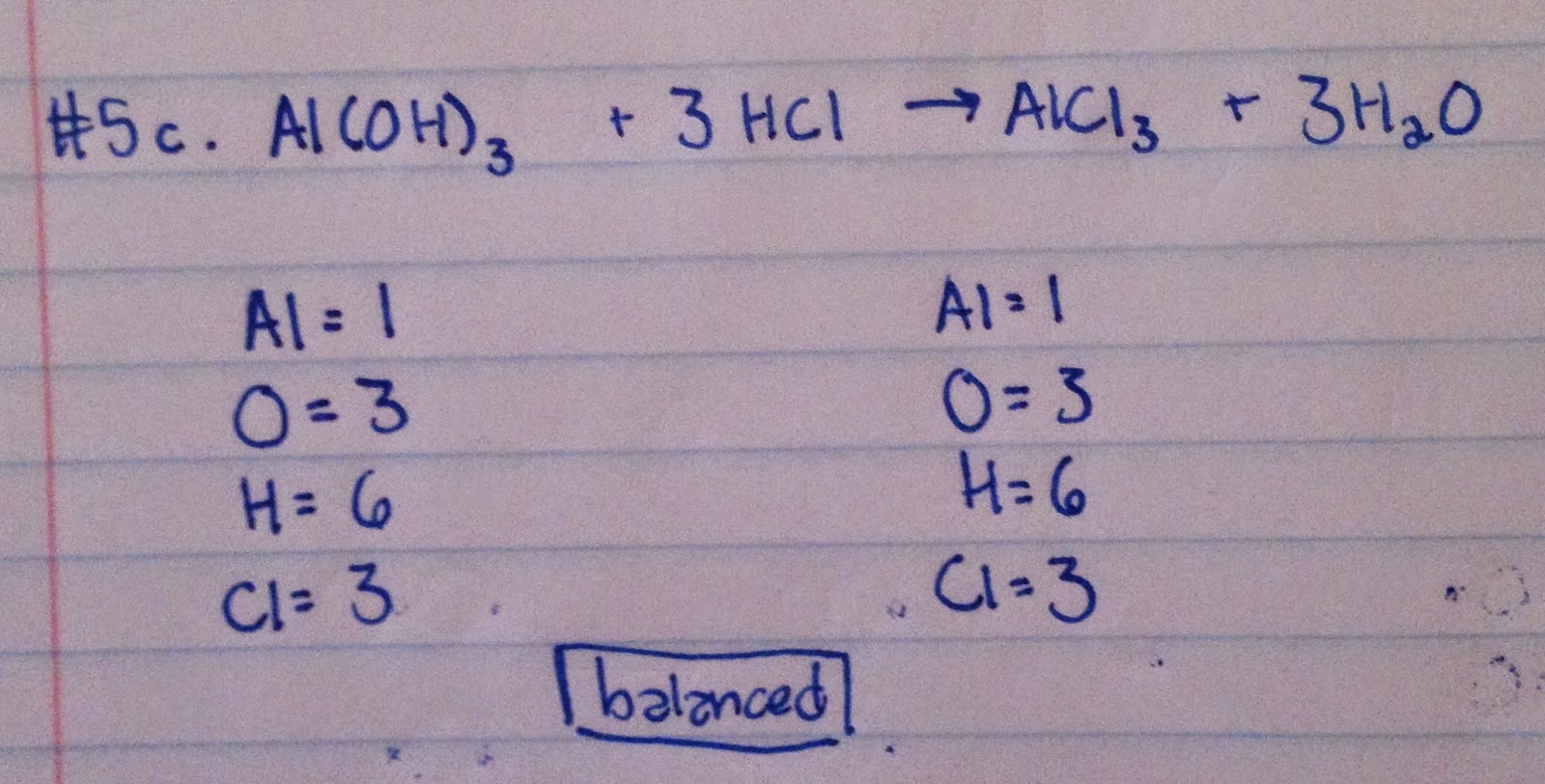#9:
a.
b.
#10:
Colloid because there are no particles.
#13:
a. 2.11%
b. 0.009%
#14:
Oceans, glaciers, water vapor, rivers.
#15:
Water is a renewable resource, because the amount of it has stayed the same for billions of years, and there's tons of it.
#16:
The water cycles through Earth's hydrologic cycle.
#1:
To remove impurities to make it drinkable.
#2:
Screening, filtration, chlorination
#3:
Removing foreign objects and substances such as oil or water or sticks.
#4:
a. Because salt water is a colloid, therefor they could not separate the salt and the water.
b. Separating the salt from the water.
#5:
#6:
Although global warming has melted some ice caps and made the water level rise, the water level of the world overall has not changed because water cannot be created or destroyed.
#7:
Being thirsty by the ocean and not having fresh water with you.
#8:
Without one step, the whole hydrologic cycle would be thrown off.
#11:
Wut.
#12:
It neutralizes acidic water.
#13:
Fluoride reduces tooth decay.
#18:
Chlorinated water is just overall safer to drink than untreated water, because the chlorine kills bacteria.
#19:
Too much chlorine in the water could be bad, and there are less of the beneficial chemicals.