#1:
a.
b.
c. (a) A molecule of hydrogen is combined with a molecule of chlorine to form two molecules of hydrochloric acid. (b) Two molecules of hydrogen peroxide are formed when two molecules of water react with one molecule of oxygen.
#2:
a. NaHCO3 + HCl ---> NaCl + H2O + CO2
b. C6H12O6 + 6 O2 ---> 6 CO2 + 6 H2O
#3:
The law of conservation of matter says that in a chemical reaction, matter is neither created nor destroyed.
#4:
A phenomenon of nature that has been proven to always occur whenever certain conditions exist.
#5:
a.
b.
c.
#6:
Expressions such as "using up" and "throwing away" are misleading because the law of conservation of matter states that atoms cannot be created or destroyed.





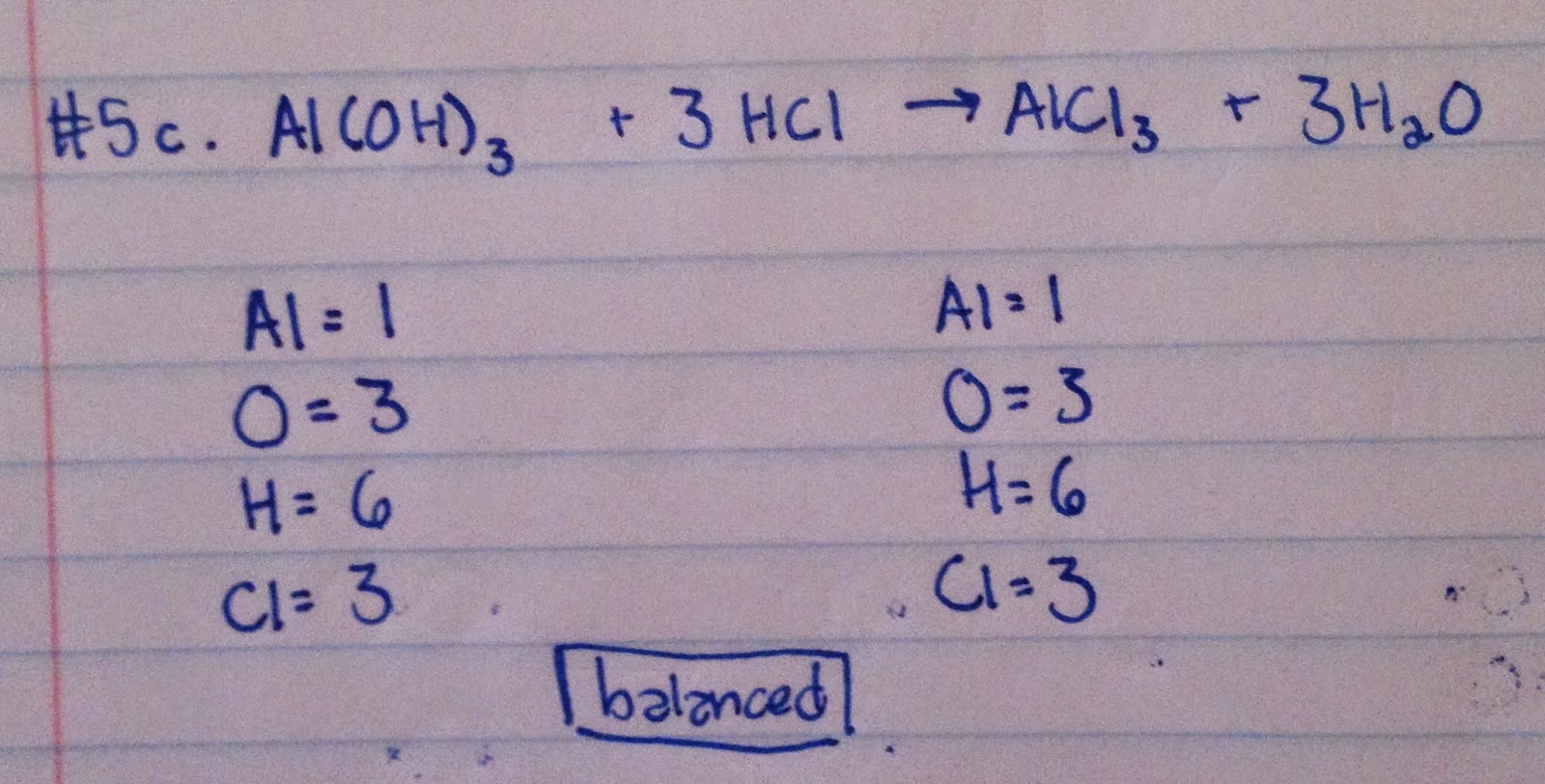
No comments:
Post a Comment