a. Two molecules of hydrochloric acid are combined with one atom of magnesium to produce one atom of hydrogen and one atom of magnesium chloride.
b. HCl is a compound, Mg is an element, H2 is an element, and MgCl2 is a compound.
c.
#1:
a.
b.
c. (a) A molecule of hydrogen is combined with a molecule of chlorine to form two molecules of hydrochloric acid. (b) Two molecules of hydrogen peroxide are formed when two molecules of water react with one molecule of oxygen.
#3:
The law of conservation of matter says that in a chemical reaction, matter is neither created nor destroyed.
#5:
a.
b.
c.
#1:
a. One molecule of methane combines with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water.
b.
c.
d.
#2:
a. One molecule of hydrobromic acid combines with magnesium to produce one molecule of hydrogen and one molecule of magnesium bromide.
b.
c.
d.
#3:
a. Four molecules of silver, four molecules of hydrogen sulfide and one molecule of oxygen combine to produce two molecules of silver sulfide and four molecules of water.
b.
c.
d.
#4:
a. One molecule of the ore hematite combines with three molecules of carbon oxide to produce two molecules of iron and three molecules of carbon dioxide.
b.
c.
d.
#5:
a. Two molecules of aluminum combine with six molecules of hydrochloric acid to produce two molecules of aluminum chloride and three molecules of hydrogen.
b.
c.
d.






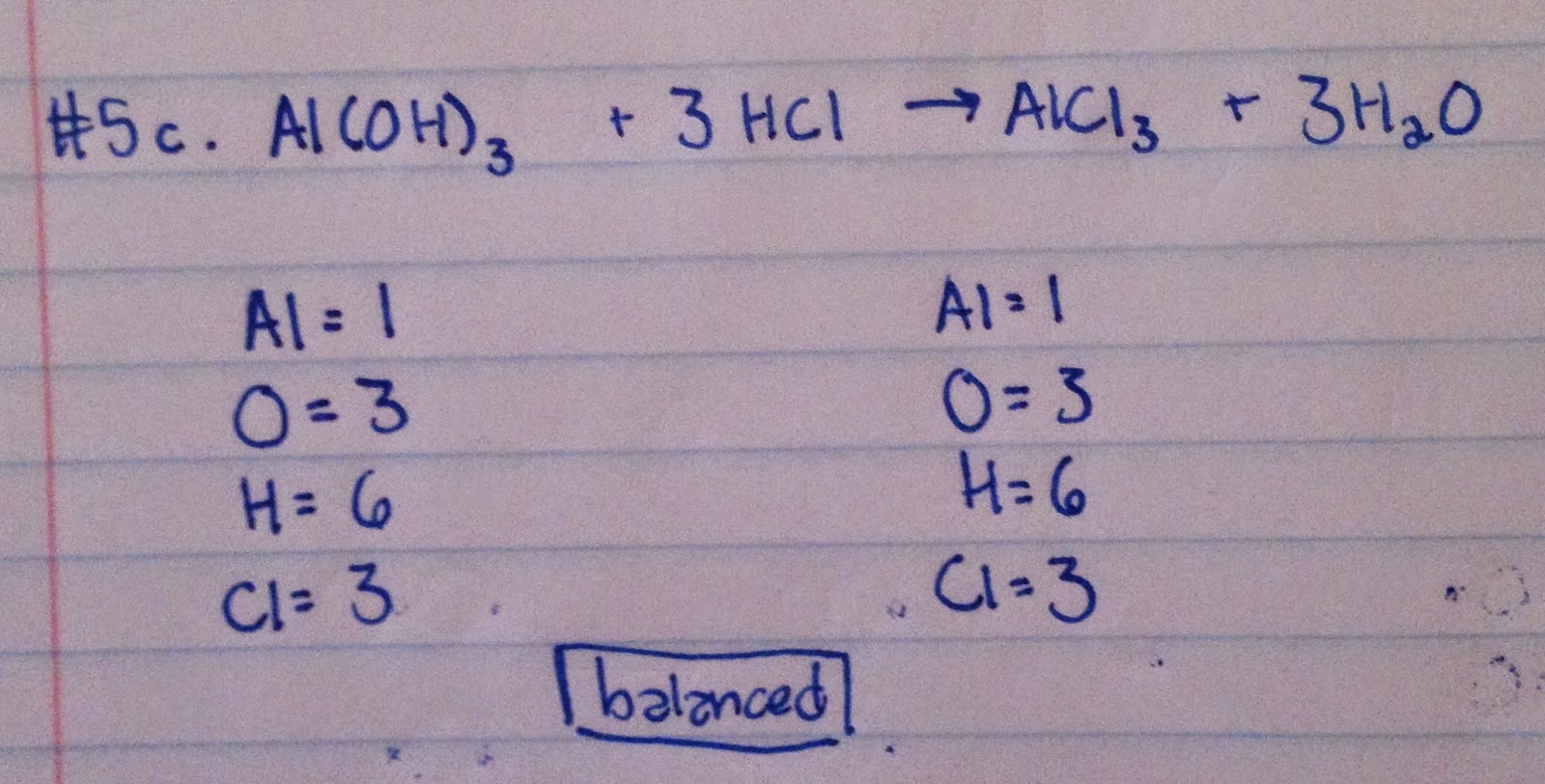















No comments:
Post a Comment